![]()
Bio. Sci. 10
Student Lecture Contributions
Summer 2008
Ethanol
by Jason Bertels
Human beings have a long history with ethanol. For thousands of years, we have been encouraging microorganism to produce it to make alcoholic beverages. More recently, we have been putting it in our cars.
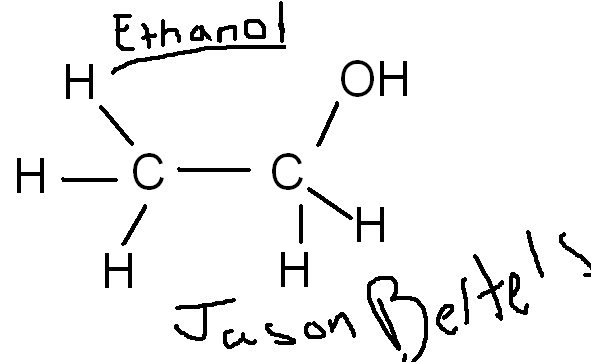
Ethanol’s chemical formula is C2H5OH. Consider the work of art below:
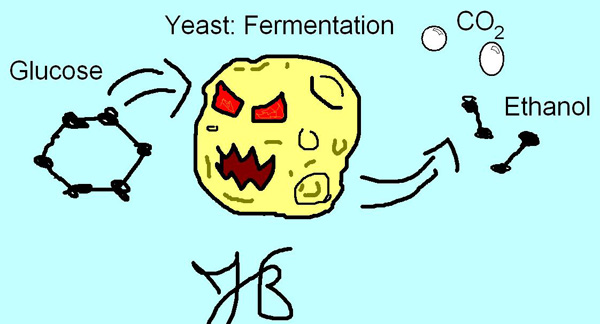
Ethanol is produced in nature through fermentation. Because we have already had an overview of this process, it seems best to concentrate only on the conversion of pyruvate (pyruvic acid) to ethanol. Pyruvate is created as a result of glycolysis, and is then converted to acetaldehyde (CH3CHO). Acetaldehyde is largely the cause of hangovers, which will be discussed later. The conversion is preformed by the enzyme Pyruvate decarboxylase, which catalyzes pyruvate, releasing CO2 and acetaldehyde. This means that this reaction starts with a 3-carbon compound and results in a 2-carbon compound. This also means that we all have pyruvate decarboxylase to thank for the bubbliness of many famous beverages and fluffy, risen bread, for the CO2 is dispelled into the environment.
The reason for ethanol production in nature is simple: to perform glycolysis, NAD+ must accept electrons and become NADH. To continue glycolysis, NADH must be oxidized. Pyruvate cannot accept the electrons, but acetaldehyde can. Acetaldehyde makes its way to the enzyme alcohol dehydrogenase, where acetaldehyde is reduced, NADH is oxidized, a proton is consumed and bonds to the forming compound, and the “final electron acceptor” ethanol is produced. Oh Joy! This website has a good diagram of it on page 6 (Warning: PDF document; I find them annoying at times):
http://bama.ua.edu/~lsbusenlehner/Chapter14b.pdf
Now that NAD+ is free to make some more pyruvate, the ethanol is nothing more than waste product. In fact, when yeast is fermenting a beverage, a concentration of about 14% ethanol will kill off the yeast. This is why yeast prefers to metabolize sugar aerobically and why distillation is necessary for higher concentrations of ethanol. Here is my depiction of the process:
Production
Because of its uses, humans are very interested in producing large quantities of ethanol. All ethanol produced for beverages is produced biologically; ethanol can also be produced for other uses through ethylene (C2H4, the monomer polyethylene, everyone’s favorite plastic) hydration (a process where a hydroxyl group OH- and a hydrogen cation (proton) are added to a carbon compound). As this is a biology lecture, we will focus on the biological methods.
Yeast consumes sugar during fermentation, however, some energy sources are starchy plants, like cereal grains. To overcome this problem, ethanol produces sometimes malt the grain. Malting is a process where the grains are allowed to germinate by soaking them in water. The tiny plants produce the enzyme amylase to convert their starchy energy reserves into sugars. The germination process is then halted by heating the grain and drying it out. When this grain, now malted, is smashed up and mixed with water, amylase continues to do its job, and the starches are converted to sugars. These sugars can now be used by the yeast.
Some common grains used for malting are barely, wheat, corn, and rice. Similar processes can also be used to convert potato starch to usable sugars.
Not all energy sources are primarily starch, however, and those that are already sugars need not be malted. Sweet fruits like wine grapes have high concentrations of sugar and are suitable for fermentation; sugar cane is also used for ethanol production.
Consumption of Ethanol
When ethanol is consumed by a human body, it is quickly passed into the small intestine, where it is absorbed into the bloodstream. It immediately acts as a suppressant on the central nervous system. Too much ethanol (BAC ≈ .4% –.5%) can suppress the vital processes in the brainstem, leading to death.
Ethanol is metabolized primarily in the liver. The first step involves one of the enzymes used in ethanol production, alcohol dehydrogenase. This enzyme is capable of working in both directions. The result is the same compound that yeast uses to make ethanol, acetaldehyde.
Acetaldehyde is the chemical behind hangovers. It is highly toxic, more so than ethanol. It causes liver damage and is a possible carcinogen. Thankfully, when the liver has to deal with only a small quantity of acetaldehyde, it almost immediately converts it to acetic acid, commonly associated with vinegar. Acetic acid is harmless. The problems associated with acetaldehyde manifest because ethanol is converted much more quickly than acetic acid can be produced. The result is a buildup of acetaldehyde. To learn a little more about hangovers, follow this link:
http://health.howstuffworks.com/hangover4.htm
Most of the acetic acid will be excreted in urine. However, the body can also catabolize acetic acid completely to obtain the energy. This is an important ability in other metabolic pathways, like lipid catabolism.
Ethanol as an Automobile Fuel
Lately, there has been a lot of interest in biofuels, including ethanol. American ethanol is mostly produced with corn, or maize. The process typically involves fermentation and distillation, until pure ethanol is produced. Then, it is mixed with gasoline, as pure ethanol is not a legal automotive fuel in the United States of America.
Another ethanol program is in Brazil. There, sugar cane is the typical source of ethanol. Sugar cane has more sugar than corn (by about 30%) than corn, and it is much more easily extracted. Brazil and the USA make up 69% of worldwide production.
Ethanol has been used to fuel vehicles for a long time. Ford’s Model T was able to be driven on ethanol, and Henry Ford touted it as the “fuel of the future.” Then, prohibition happened, and police officers around the country destroyed domestic ethanol stills (some of which were for lamp and heating fuel, not consumption), and the absolutism of prohibition prevented any advancement in the ethanol-fuel industry. When prohibition died, the staus quo was pure-gasoline, in contrast to other countries like France, where gasohol (ethanol and gasoline mixture) was mandated. Intrest was sparked in the 70’s oil crunch, and again in the new millennium.
The Energy Policy Act of 2005 legislates that the USA produce 7.5 billion gallons of ethanol a year by 2012. We are currently producing about 7.0 billion gallons a year. To put this into comparison, the US gasoline market is about 150 billion gallons.
One may ask, “what are the costs?” One cost is gasonine: ironically, the corn grown for domestic ethanol is farmed with gasoline, and the net energy gain is slight according to some sources, and negative according to others. Many studies, including one from Cornell University and UC Berkley state that corn ethanol uses 26% more fossil fuel energy than it replaces. Another cost is food. Currently, ethanol is only produced commercially from footstock. This is exacerbating poverty situations all over the world, as it takes about 26 pounds of corn to eventually produce 1 gallon of ethanol. Jean Ziegler has been the United Nations' expert on food rights since 2000, and has called for a 5 year moratorium on ethanol production, until methods are developed to economically produce ethanol from non food sources, like cellulose. There has been headway in this area, as the first cellulose-to-ethanol facility opened in Canada in 2004, operated by Iogen Corp., but is still in the demonstration phase. The hope is that the next generation of ethanol production facilities will process what amounts to garbage (corn cobs, sawdust, etc) instead of valuable food. UN predictions forecast widespread famine if the current expansionary ethanol trend continues. In fact, Mr. Ziegler calls current production methods a “crime against humanity.”
As exemplified with Prohibition, politics seem to dictate the global ethanol trends (as opposed to economics). As ethanol producers receive subsidies, the yearly output continues to increase.
Ethanol: The Next Generation
Advances continue to be made with respect to ethanol. For example, new ethanol producing microorganisms are being discovered, including Clostridium ljungdahlii, which can synthesize ethanol from gases creasted by incomplete buringing of organic matter, carbon monoxide (CO) and hydrogen gas (H2). Ethanol is also a prospective fuel- cell fuel. As continued research by biologists and other scientist progresses, we should see new and exciting things for the product of humanity’s first controlled organic reaction.
References
Wikipedia. <http://www.wikipedia.org>.
“BIOL 103 Lecture 26.” Accessed 19 June 2008. <http://webs.wichita.edu/mschneegurt/biol103/ lecture26/lecture26.html>.
Prof. Shakhashiri. “Ethanol.” 23 Jan 2008. Accessed 19 June 2008. UW Madison. <http://scifun.chem.wisc.edu/CHEMWEEK/PDF/Ethanol.pdf>.
Clayton, Mark. “The politics of ethanol outshine its costs.” Christian Science Monitor. 15 Nov 2007. Accessed 20 June 2008. <http://www.csmonitor.com/2007/1115/p02s02-uspo.html>.
Lang, Susan S. “Cornell ecologist's study finds that producing ethanol and biodiesel from corn and other crops is not worth the energy.” Accessed 20 June 2008. <http://www.news.cornell.edu/stories/July05/ethanol.toocostly.ssl.html>.
Lederer, Edith M. “UN Expert Calls Biofuels ‘Crime Against Humanity’.” 27 October 2007. Accessed 20 June 2008. <http://www.livescience.com/environment/071027-ap-biofuel-crime.html>.
Copyright 2008 Sierra College Biological Sciences Department. All Rights Reserved.
![]()
![]()